Meteorologists may say clouds are all about weather, but they sure aren’t, or at least not all of them! This late winter, people watching the sky in northern England witnessed spectacular appearances of nacreous clouds, also referred to as mother-of-pearl clouds (see Figure 1). Nacreous/mother-of-pearl is used to describe the inner, hard, and iridescent layer of certain seashells, and that is exactly how nacreous clouds in the sky look – shining like pearls. The more technical name is polar stratospheric clouds (PSCs), which tells us where these clouds can be found. PSCs are located in the polar stratosphere at altitudes between 15 and 25 km. They become visible to the human eye when the Sun is below the horizon, with sunlight being reflected from the clouds at very low angles. PSCs form at very low temperatures (below -78° C), encountered in the polar stratosphere only during winter. The main reason they occur at such low temperatures is the very small water vapour concentrations in the air (only 5 out of 1 million air molecules are water molecules). Despite their beauty, however, PSCs have a nasty side to them — they can severely enhance stratospheric ozone loss. This is the reason for the Antarctic ozone hole.
Figure 1: Polar stratospheric clouds (PSCs) as photographed in January 2016 near Tromsø, Norway. (courtesy Geir Braathen, World Meteorological Organization, Geneva). More PSC photographs on the Cloud Appreciation Society website
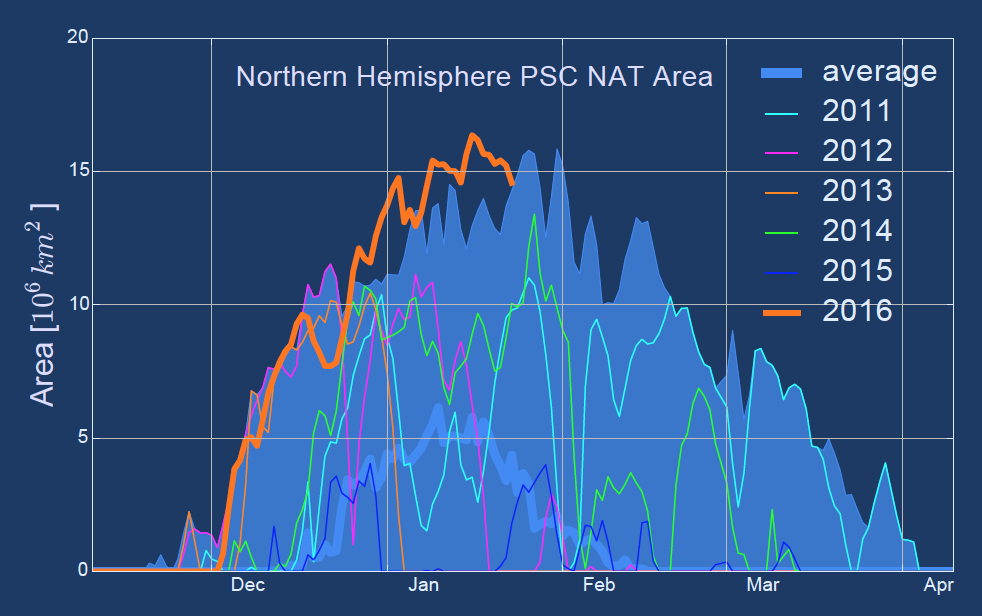
Chemically, there are two different types of PSCs. Type I PSCs form at temperatures below -78° C and consist of nitric acid trihydrate (NAT = [HNO3•3*H2O]) particles. Type II PSCs form at even lower temperatures (below -85° C) and consist of pure water-ice crystals (H2O). The formation rate is also dependent on the availability of condensation nuclei such as liquid sulphuric aerosol or meteoritic material. Volcanic eruptions that lead to the injection of sulphur oxides into the lower stratosphere for example can strongly enhance the availability of such condensation nuclei and hence the formation of PSCs. Because temperatures in the southern hemisphere polar winter stratosphere are generally lower than in the northern hemisphere, PSCs occur more extensively in the southern hemisphere. This northern hemisphere winter has, however, seen temperatures much below the PSC-type I formation threshold value from early December through January, extending over an area of more than 15 million square kilometres at times (see Figure 2).
Figure 2: (upper) 50°N-90°N Arctic minimum temperature at 50 hPa averaged over 1979-2014 (light grey) with 70% and 90% percentiles highlighted in light and dark turquoise. Coloured lines show different years, with red being the winter of 2015/16. (lower) Integrated area where PSC formation is possible in the Northern Hemisphere. Here the average is in blue, the 2015/16 winter in orange. (WMO Bulletin, 2016)
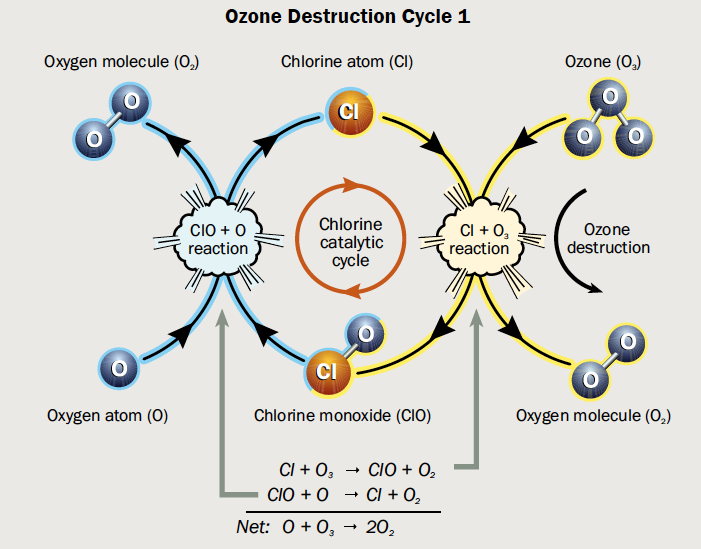
It’s on the surface of PSCs that chemical reactions occur in which reservoir species of chlorine (such as HCl and ClONO2, which are not reactive enough to be involved in ozone depletion) are converted into Cl2. In spring, with the help of sunlight, Cl2 is split into single reactive chlorine atoms (Cl). Cl is involved in the catalytic cycles that lead to efficient ozone destruction, with one Cl atom being estimated to destroy up to 100,000 ozone molecules over its lifetime (see Figure 3).
Figure 3: Catalytic cycle of chlorine (Cl) that leads to ozone (O3) destruction. In a first step, a chlorine atom reacts with O3, forming a chlorine monoxide (ClO) and an oxygen molecule (O2). In a second step, the ClO reacts with an oxygen atom (O), regenerating the Cl and thereby starting the cycle anew. (Hegglin et al., WMO 2015)
Given the large area in the polar stratosphere over which PSC-formation temperatures have been recorded this year, and extensive observations of PSCs in the Northern sky, the big question for stratospheric ozone scientists now is, will we experience a record-ozone hole over the Arctic once the sunlight returns in spring?
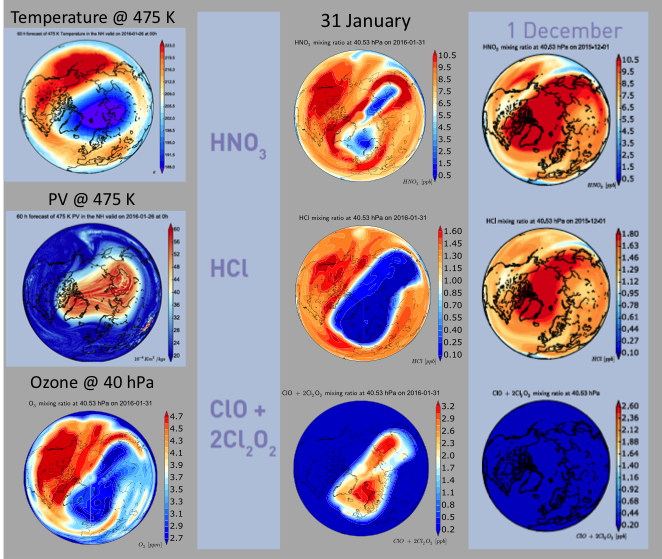
The question has no definitive answer as yet, which is why a whole army of scientists is currently in the polar circle with their instruments ready to measure ozone depletion, while others are eagerly studying meteorological forecasts. The key ingredient to the development of severe ozone loss is in fact meteorology. It will be crucial that the air masses that experienced activation of Cl2 remain isolated from mid-latitude air until they see sunlight that will form Cl and start the catalytic destruction cycle seen in Figure 3. If mid-latitude air is mixed into the polar regions too early then the chlorine atoms can get deactivated by reactions involving nitrogen oxides (NOx) or hydrogen oxides (HOx) before ozone destruction can take place. Continued isolation of the polar air masses will occur if the polar vortex (strong circumpolar winds delineating the area of low temperatures and high potential vorticity, see Figure 4) remains intact for another couple of weeks. While a stable vortex is currently still present (and some ozone depletion has already taken place), forecasters see some probability of rising temperatures and even a sudden stratospheric warming, which could lead to rapid decay of the vortex. Let’s keep our eyes on the sky!
Figure 4: (left) ECMWF analysis of temperature and potential vorticity (PV) on 26 January 2016, along with ozone and (middle) HNO3, HCl, and ClO+2Cl2O2 maps at around 40 hPa on 31 January 2016. Air depleted in HNO3 and HCl within the polar vortex region are a sign of chlorine activation (as seen in enhanced ClO+2Cl2O2 values in lowermost panel). (right) For comparison, corresponding constituents maps on 1 December – reflecting conditions before chemical reactions on PSCs have taken place – are also shown. (courtesy Geir Braathen, World Meteorological Organization, Geneva)