These past two weeks I have realised two things:
- The gym is no more appealing in 2013 than it was in 2012
- The vast majority of scientists can be separated into two types: ‘outdoor’ and ‘indoor’ ones
Outdoor scientists bring the outdoors into their labs. I am, and have always been an outdoor scientist. Scientists who fall under this category don’t usually wear lab coats and don’t really understand when one is really needed (if they do own a lab coat it is likely either to be the one they haven’t used since Chemistry GCSE classes or a grimy, brown monstrosity that is used to protect their clothes from getting dirty). Outdoor scientists have ‘dirty’ labs where the lab fridge can harbour all manner of things from pots of sleepy spiders (to cool them down and make identification easier) to soya milk (for tea, obviously). The tiny freezer section of a dirty lab’s fridge only ever becomes filled in summer, when it’s crammed with icecream. A group of outdoor scientists in a lab will complain about the choice of radio station.
Indoor scientists try and keep the outdoors out of their labs. They know when to wear lab coats and when to use blue gloves instead of plain latex ones. There’s no food fit for human consumption to be held in an indoor scientist’s fridge. Instead, Petri dishes wrapped in sterile tape are stacked neatly on top one another. The freezers are full of candy-coloured boxes of samples. Needless to say, you won’t find any candy (or Ben&Jerry’s) here. A group of clean scientists in a lab will complain about their pipetting repetitive strain pain.
I have spent the last two weeks in the pathology lab at Wisley. For obvious phytosanitary reasons, it is (and needs to remain) a clean lab. I was there to prepare my nectar samples before sending them off to be analysed at Newcastle University.
In earlier posts (here and here), I mentioned how, in a collaborative project between the RHS and Newcastle University, I had been sampling everything that flowered on our Plants for Bugs plots on five occasions between April and September.
My first attempt at sampling nectar from the garden in April 2012
The nectar was collected in tiny glass tubes (microcapillaries) and then frozen.
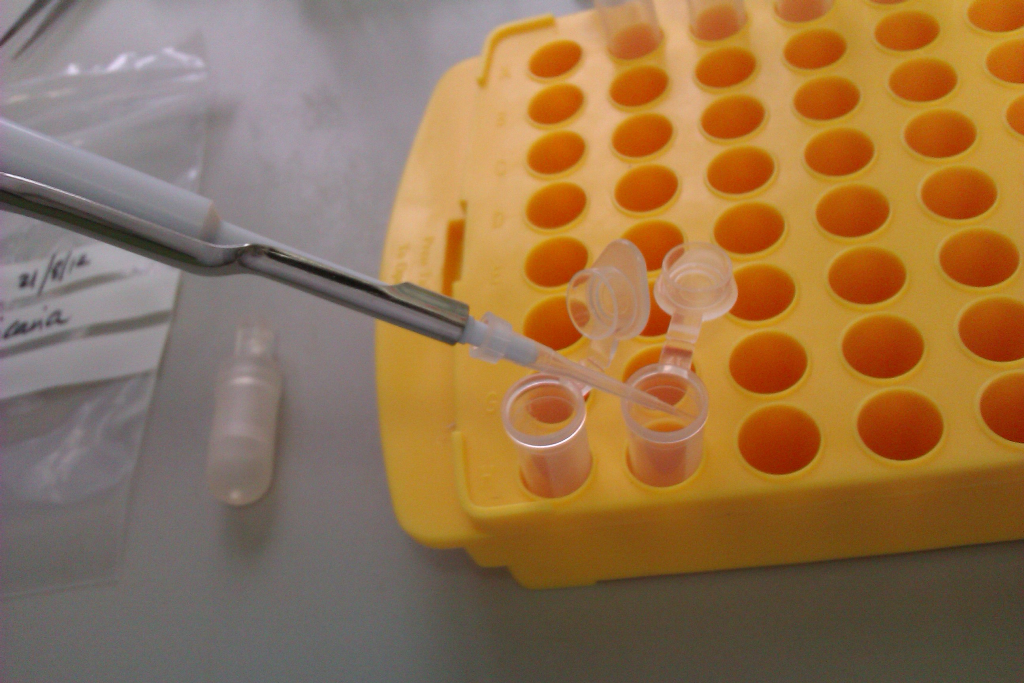
Having completed sampling for 2012, I now needed to prepare each sample for HPLC analysis by diluting with very pure water. This involved expelling nectar from each of the 2000+ microcapillary tubes into 1.5ml plastic, lidded tubes called eppendorfs. (Q. what’s worse than getting nectar into a microcapillary tube? A. Getting nectar out of a microcapillary tube.)
Left: Using a squeegee to expel nectar from a microcapillary tube into an eppendorf. Right: the tiny quantities of nectar from the microcapillary visible as a spray on the inner side of the eppendorf.
The last step involved diluting the nectar to ensure there was sufficient volume for each sample to be analysed by the HPLC machine.
Four hundred and fifty samples later, we are now ready to send off our prepped samples. So, as part of my KTP with the University of Reading, it seems I have also learnt a great deal more about the very cool gadgets that you can find in an indoor scientist’s lab. Whilst I’m still unsure on the rule regarding when to wear a lab coat (I will continue to don mine as soon as I cross the threshold until I hear otherwise), I’m pleased to say I now know the difference between blue and white gloves (blue ones much more expensive) and can hold my own in a discussion about pipetting wrist ache.






Out of interest (as a fellow outdoor scientist – woo, plant ecology!), approximately how many lab hours did it take you to dilute those 450 samples for HPLC? I’m doing similar work, and have a penchant for knowing how long tasks could/should be taking me.
Thanks!
Hi Jo,
Lovely to hear from someone doing similar work! I’d say it took me roughly 40 hours to prepare all 450 samples. It will probably take you significantly less time as it took me a little while to get used to the lab equipment and to perfect the protocol. The longest step was making sure I got all my nectar out of each eppendorf!