From Sediment to Slides – phytolith training at the University of Exeter
For the past few weeks I have been lucky enough to have been working in the Archaeology Department at the University of Exeter, where I have been learning how to analyse soil and sediment samples for phytoliths.
In case you are not familiar with them, phytoliths are plant opal silica bodies that are formed from monosilicic acid (H4SiO4) taken up by the plant that is then deposited in the cells of the stems, leaves, roots and inflorescences. These silica bodies are formed in different shapes and sizes depending on the type of plant and where in the plant they are produced. In some plant groups, such as Poaceae (grasses), the phytolith characteristics are diagnostic to the sub-family level. You cannot identify these sub-families by analysing pollen grains alone, so phytoliths add another level of detail to palaeo-vegetation reconstructions. Additionally, phytoliths allow you to work at a fine spatial scale because when plants decay the phytoliths are deposited in the soil where the plant lived, so they represent the local vegetation. They also preserve well in aerobic and acidic conditions, so they can be recovered from soils as well as lake sediments.
I spent my first week in Exeter learning how to process soil samples for phytolith analysis with Jenny Watling. From soil to microscope slide it can take about 5 days, depending on the size of your sample. A lot of that time is spent repeatedly washing your sample in the centrifuge after each treatment. Essentially, you need to: (1) remove the clays, (2) remove the carbonates, (3) remove the organics, (4) retrieve the phytoliths by floating them in a heavy liquid, and (5) dry your phytoliths ready for mounting onto a microscope slide.
Once the slides are ready, you can count the different types of diagnostic phytoliths and draw conclusions about the vegetation that produced them. The key to this is to spend a long time looking at modern reference collection slides to learn what to look out for, and of course having helpful experts on hand to point you in the right direction – thank you Josѐ, Jenny, Sheahan and Lautaro! I spent many hours photographing the extensive reference collection from Dr Josѐ Iriate’s lab to form my own mini digital collection to use when I returned to Reading.
All photographs courtesy of Dr Josѐ Iriate, Department of Archeology, University of Exeter
In my final week the really interesting work began as I started to analyse my samples from a lake sediment core taken by Dr John Carson from an ox-bow lake in Acre state, Brazil. My first experience of analysing a real sample slide was quite daunting; it took me almost 5 hours to count 100 phytoliths. Thankfully it turned out I had chosen an unfortunate sample for my first analysis, with a very low concentration of tiny phytoliths, and my second attempt was much more successful; 200 phytoliths in 2 hours.
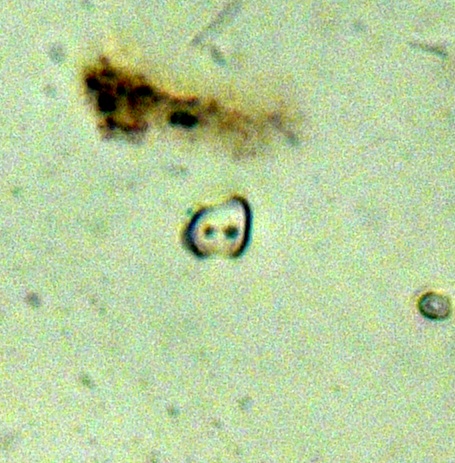
 Picture 11: Image from a slide showing a scooped bilobate (Erhartoideae), a panicoid-type bilobate, a tall saddle (Bambusoideae) and a globular granulate (arboreal indicator)
Picture 11: Image from a slide showing a scooped bilobate (Erhartoideae), a panicoid-type bilobate, a tall saddle (Bambusoideae) and a globular granulate (arboreal indicator) Picture 12: Image from a slide showing two crosses (Panicoideae) and two echinate globulars (Arecaceae)
Picture 12: Image from a slide showing two crosses (Panicoideae) and two echinate globulars (Arecaceae)
I have learnt a lot in my time in Exeter and thoroughly enjoyed it. Now I’m excited to start working on pollen as well…
Heather Plumpton,
PhD student at the University of Reading
Meet Heather:
In my PhD with the Tropical Palaeoecology Research Group at the University of Reading I am interested in looking at ecological patterns over long timescales. I will use palaeoenvironment proxies, such as fossilised pollen and phytoliths, to reconstruct vegetation responses to climatic changes. In particular, I am investigating the long-term impacts of a mid-Holocene (~6000 years ago) drought on ecotonal regions of the Amazon rainforest in northern Bolivia. I am also interested in how plant-climate interactions are represented in Dynamic Global Vegetation Models (DGVMs) and how this feeds into Global Climate Model predictions for future plant responses to climate change.