Dracaenaceae Salisb., Gen. Pl: 73 (1866), nom. cons.
The family Dracaenaceae has a complex, fascinating history, of great interest will be to see how it unfolds. It is the family of Dragon Trees, of one, two or three genera and a hundred or more species, of mythical properties and misplaced relationships. For most of this history it’s maintained an internal constancy, as Dracaena and Sansevieria, but there are several other tree-like, rosette-topped, spiky leaved, monocot genera with which it has either primitive or derived similarities and has been variously combined. How then has it come to be thought of by some as part of the subfamily Nolinoideae, again placed in the worldwide family Asparagaceae?

D. cinnabari, a forest of Dragon Trees.
© Image courtesy of Jan Vandorpe
Draceana, from the greek word “drakainia”, meaning female dragon, is the origin of the family name. Dracaena is associated with dragons through the Greek Heracles myth, his 11th labour was achieved by slaying a hundred-headed, Dragon Tree dragon. Pégot-Ogier (1871) describes how in legend the Dracaena draco tree in question grew to huge proportions on Tenerife, and was seen in its full glory by such famous explorers as Humboldt who, in the first half of the nineteenth century, declared it to be already ten thousand year old. The photograph here, of the Dragon Tree considered by Arnoldo Santos to be the oldest still growing on Tenerife, was taken by the more recent explorer, Alastair Culham, in Orotava, where the original reputedly grew. A descendent? Regrowth?
Several species also exude a deep red resin known as Dragon’s Blood when their wood is cut or wounded. One is Daemonorops draco, not related to our plants, a rattan-palm from South-East Asia, in the West Indies the papilionoid legume Pterocarpus draco is the source, from Mexico it’s Croton lechleri and the Venezuelan’s have Croton gossypifolium. Our sources, reputedly the original ones, are Dracaena cinnabari, endemic to Socotra, D. draco of the Canary Islands, recently found too in Morocco, and from China, D. cochinchinensis. Today, the main commercial source of Dragon’s Blood is Daemonorops (Gupta et al., 2008).

The leaf bases of Dracaena cinnabari, showing the colour of the resin which exudes when they are removed or the bark is cut. RBG Kew, Feb. 2013.
Dragon’s Blood has been prized continuously since the early Greeks as a panacea and imbued with mythical properties. There is now evidence that it has antibacterial, anti-virus, anti-carcinogenic, anti-diarrhetic, anti-ulcer, anti-tumor, anti-inflammatory, antioxidant and wound-healing properties, of great potential, which haven’t yet been fully investigated. Gupta et al., 2008.
- Resin collected from D. cinnabari, Socotra. © Image courtesy of www.socotra.info
- D. cinnabari, raw resin exudate, dried. Socotra. © Image courtesy of www.socotra.info
- Powdered D. cinnabari resin, prepared for re-sale, probably medicinal use .© Image courtesy of www.socotra.info
- The leaf bases of Dracaena cinnabari, showing the colour of the resin which exudes when they are removed or the bark is cut.
In the middle-ages, over-collection of D. draco for its medicinal benefits led to it becoming scarce. The IUCN Red List, IUCN 2013, now records that it’s Vulnerable (VU A1abcde), meaning that it has previously declined significantly and measurably but that the decline has now ceased. D. cinnabari has a status of VU (Vulnerable B2ab(iii)), reflecting its extremely restricted distribution and a continuing threat of decline. IUCN list eight other species of Dracaena, one as Extinct, five are Endangered, two more are Vulnerable and one Data Deficient. Five Pleomele species are also listed, three Endangered and two Vulnerable; all are Hawaiian species, which Jankalski places in Dracaena subgenus Chrysodracon and into synonymy in some cases, but this is getting ahead of the story ….

Dracaena and Sansevieria spp. and cultivars as House / Office / Shop plants
Sansevieria trifasciata ‘Laurentii’ © Happy Sharry, on Flickr; Dracaena marginata © blumenbiene, on Flickr; Office Plants © flyover, on Flickr; CC Licensing.
As well as having medicinal value, it is by virtue of great patience with neglect and tolerance of a wide-range of plant-inhospitable environments, a ubiquitous house and office plant. D. marginata, with its reddened leaf margins, is the main species used. Given its ubiquity in this sense, there seem to be remarkably few cultivated varieties available on the market. For example, Plants in Buildings, 2012, list only 15 species and varieties of Dracaena and 3 varieties of Pleomele. Sansevieria, on the other hand, has attracted a following and is said by the International Sansevieria Society, 2012, to have 130-140 species and cultivars. Sansevieria is also a common house plant, familiar to us as Mother-in-Law’s Tongue and is renowned as a valuable source of fibre, for which it has long been cultivated in parts of Africa.
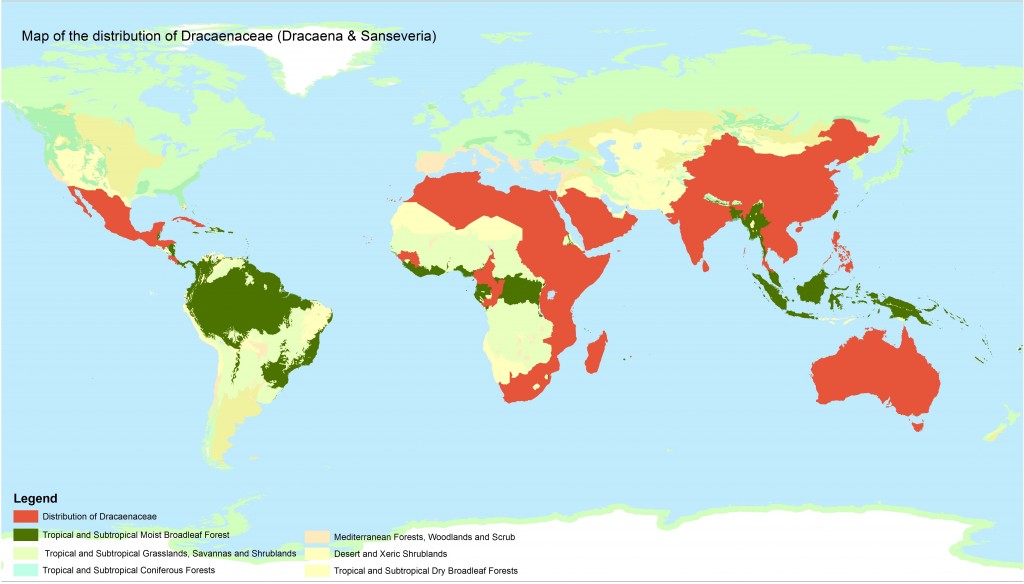
Distribution Map of Dracaenaceae (Dracaena and Sanseveria) N.B. this is a representative map by countries identified by the sources below. It is also said to occur in suitable climates in other African and Asian countries. It does not occur in South America. Suitable biomes are shown.
Sources, Jankalski, 2008; Marrero, 2008; Brown, 1915.
The Genera
Any family circumscribed to include Dracaena has also included Sansevieria and Pleomele, sometimes sunk in Dracaena itself, sometimes not. These one, two or three genera constitute a “core” Dracaenaceae.
Dracaena Vand. ex L.
The type genus, when the family Dracaenaceae is recognised. D. draco (L.) L. is the type species.
Comprises 121 species currently accepted, WCSP, 2013, 2 subsp. and 15 varieties.
Published estimates vary, sometimes in error, Heywood et al., 2007 and APG III+, for example, give about 100 species, but their extent includes Sansevieria and Pleomele and so is an underestimate. This is confirmed by Dahlgren et al., 1985, for example, who reports 80-150 species of Dracaena and 50 of Sansevieria.
Dracaena is arborescent, made so, unusually for the monocots, by secondary thickening arising from a meristematic zone in its stems, which develops typical scattered bundles. Trunks reach more than 2m in diameter in some species, Dahlgren et al., 1985. The roots have xylem vessels, which is also very unusual and equally diagnostic. Dracaena and Sansevieria have resin canals in their bark and leaves which often produces the totemic red- or orange-staining resin, Judd et al., 2008, but only on “dog days”, Pégot-Ogier, 1871.
Figure 2. Dracaenaceae: characters to separate Dracaena from the other genera.
Dracaenaceae: genera
(Right click to open in a new tab)
Only six species achieve tree size, D. draco. D. cinnabari, D. ombet, D. schizantha, D. serrulata and D. tamaranae, all with North or East-African distributions, the rest are shrubs, or occasionally acaulescent. If Pleomele is included, this number gets larger, as some Hawaiian species qualify (Marrero, 1998). Heywood et al., 2007 say that Dracaena can reach to 40m in height, this follows Bos, 1998 and appears to be a mistake. Mabberley, 1987 and many other authors take 20m to be the tallest, and even the largest tree of D. draco until comparatively recently on Tenerife, one with a mythology all of its own, including its age of 4,000 years, is only said to have reached 18m, with, however, a trunk width of 18m and a canopy spreading 200m, Pégot-Ogier, 1871. They’re long-lived, but as monocots, can’t be aged by annual rings.
Description, morphology and more …..
Dracaena Vand. ex L.
- Dracaena draco. Note this is the famous, mythologically associated tree from Tenerife. Habit, flowers and fruit. Curtis’ Botanical Magazine, Vol. 27 via BHL.
- Dracaena draco. Flowers and fruit, expanded, with explanatory caption. Flowers +- 2cm across. Curtis’ Botanical Magazine, Vol. 27 via BHL.
- Dracaena (and Pleomele) flowers, detail showing the structure (an the differences used previously to distinguish Pleomele). © RBG Kew, Brown 1914 (with annotations).
- Dracaena draco. Leaf rosette.Leaves +- 40cm. Herbarium RNG, February 2013.
- Dracaena draco. Leaf bases, showing dried resin. Bases +- 5cm wide.Herbarium RNG, February 2013.
- Dracaena draco. Characteristic berry-like fruit. Berries +- 1cm. Herbarium RNG, February 2013.
- Dracaena draco. Characteristic berry-like orange fruit. Berries +- 1cm. Herbarium RNG, February 2013.
- Sanseveria longiflora. Showing an elongated perianth tube, not characteristic of this genus. Curtis’ Botanical Magazine, via BHL.
- Dracaena schizantha. Fresh fruit ripening. RBG Kew, Jan. 2013
- Dracaena schizantha. Fresh resin on leaf bases. RBG Kew, Jan. 2013
- Dracaena schizantha. Branching. RBG Kew, Jan. 2013
- Dracaena schizantha.Top of stem and leaf bases. RBG Kew, Jan. 2013
- Dragons found in Morocco. D. draco ssp. ajgal, only named in 1997. Here growing in the UoR greenhouses.
- D. draco subsp. ajgal, showing the propensity of the species for producing red resin at the leaf bases. Here growing in the UoR greenhouses.
Sansevieria Thunb. nom cons.
Comprises 73 species currently accepted, WCSP, 2013, and 7 varieties. Sometimes spelled Sauseverinia, Sanseveria, or Sanseviera, and, sometimes attributed to Petagna, the authorship is a murky tale in itself, Brown, 1915.
Bentham and Hooker, 1883 placed Sansevieria in Haemodoraceae with the Kangaroo Paws, the only arrangement under which these genera have been in different families. It was moved to Liliaceae by Engler in 1887, Brown 1914.
The literature oscillates Sansevieria into and out of the synonymy of Dracaena. It is likely to be this taxonomic confusion that is responsible for the differently reported species numbers of Dracaena and Dracaenaceae. Opinion is still split, with e.g. Heywood, 2007 and APG III+ combining them, while WCSP 2013, and hence Kew’s The Plant List, 2010, retain them, on the evidence of Jankalski, 2008. Bos, 1998 affirms that Sansevieria is held to be separate from Dracaena only by tradition and gives examples of Dracaena, D. ombet and D. braunii, that show Sansevieria characteristics of acaulescence, therefore he treats it as a synonym of Dracaena. APG III and other authors such as Lu and Morden (2010) justify combining, the genera however, this paper does not treat the position of Sansevieria.
Bos’ evidence is strongly contradicted by Marrero et al., 1998 and others, who refer to D. ombet as clearly having a trunk. This synonymisation was also rejected earlier by Brown, 1914, who emphasises their different perianths (see Figure 2) and concludes that Sansevieria should be retained, which separation lays open his path to re-recognising Pleomele, which is vegetatively closer to Dracaena, mostly caulescent, but has Sansevieria perianth tubes. The molecular phylogenetic work of Bogler et al., 1996, finds them to be close, and monophyletic, but Bogler does not propose they are combined. Jankalski, 2008 citing his own earlier work (not seen) also dismisses the merger, on seed and pollen characteristics.
It is not clear from the evidence cited that the case for combining these genera is substantial, and it seems that some authors have based treatments on earlier ones without necessarily having evidence; whereas there seem good morphological and practical reasons to maintain the two genera.
Pleomele Salisb.
This genus has gone curiously unnoticed by some authors. Bos, 1998 who justifies his opinion that Sansevieria should sink into Dracaena, places Pleomele likewise, but without evidence, though he is clearly aware of Hawaiian Dracaena species and therefore of Pleomele. Seberg (in Heywood et al., 2007) also says nothing about Pleomele, nor does the published APG II or APG III. APG III+ mutters at it, citing “e.g.” Lu & Morden 2010 by way of reason for its inclusion in Dracaena. The evidence held in the “e.g.”, seems significant – as might be the search for it through their hundreds of references – given the lightness of Lu and Morden’s conclusions.

Pleomele auwahaiensis St. John, habit,
(D. rockii (H.St. John) Jankalski)
© Forest & Kim Starr, CC Licensing

Flowers of Pleomele auwahaiensis St. John, inflorescence,
(D. rockii (H.St. John) Jankalski)
© Forest & Kim Starr, CC Licensing
The molecular evidence from trnL-trnF and psbA-trnH genes extracted by Lu and Morden, 2010, does show the sampled Pleomele species are monophyletic with Dracaena, but the results are not entirely consistent and some bootstrap values are low, despite retaining all gaps so bolstering their findings. Their sample of Pleomele comprises 5 Hawaiian endemics from subgenus Chrysodracon and only one species of subgenus Pleomele, sensu Jankalski (see below), samples of more species of typical Dracaena are needed to confirm this result.
Morphological evidence for the demise of Pleomele is provided by Jankalski, 2008, but by way of reason he states only that “it is now widely accepted”. He proceeds to publish Pleomele as a subgenus of Dracaena, using several strong-looking characters, detailed in Figure 2, to retain this separation, and at the same time names subgenus Chrysodracon to include all of the Hawaiian Pleomele species.
Long previously, Brown, 1914, studiously justifies not only the retention of Pleomele, but proposes it comprise, almost entirely from Kew material and therefore a subset of eligible species, 93 species previously of Dracaena. He retains only eight species in Dracaena, cheeringly two are the Dragon’s Blood donors, D. draco and D. cinnabari.
Given that WCSP cites Jankalski as the reason to combine Pleomele into Dracaena, what grounds were there for APG III and other authors to do so, who do not cite this paper? There is the unconfirmed, post-hoc evidence from Lu & Morden, and the unexplained, frequently cited comment from Bos, however, this does appear to be an assumption based upon a truism. Some species are quite distinctive ….

Pleomele aurea (H. Mann.) N.E. Brown (Dracaena aurea (H. Mann))
© Image courtesy of Karl Magnacca.

Pleomele aurea (H. Mann.) N.E. Brown (Dracaena aurea (H. Mann))
Flowers.
© Image courtesy of Karl Magnacca.
To follow the Dracaenaceae story, it will help to understand a little bit about the characters, namely the genera once or currently considered to be closely related to it, particularly those significant in the delineation of the families and subfamilies in which it has from time to time resided. These accounts should be read in conjunction with Figure 3, where more detail about the family history, systems and delineations is provided.
Figure 3. Dracaenaceae: history of the family and its placement in various systems of classification .
Dracaenaceae: family history
(Right click to open in a new tab)
Agave L.
Agave nicely illustrates the early classical familial thinking of Bentham & Hooker, 1883 and Engler and Prantl 1897. Agave with its inferior ovary they placed in Amaryllidaceae, whereas Dracaena was regarded as liliaceous and so placed there with other superior ovaried genera, like Convallaria, Asparagus, Ruscus, Nolina and Yucca.
Dracaena was included in the Agavaceae, for example by Hutchinson, 1973, based upon shared arborescence. Both Dahlgren et al., 1985 and later Bos, 1998, who follows Dahlgren’s classification closely, dismiss arborescence as convergent in these families, but characteristic at the Order level, of Asparagales, compared to the mainly herbaceous Liliales, which are also divided by combining sixteen diagnostic characters.
Dahlgren et al., 1985, in a controversial treatment of the Liliaceae, recognised small, homogeneous and purportedly monophyletic families from a number of the key genera, including Agavaceae and Dracaenaceae. Among the many characters used to define the families, most consistent is the presence or absence of a dark outer seed-coat of “phytomelan”. The order contains families with superior and inferior ovaries.
Convallaria L.
Affinity of this genus with Dracaena was found in early phylogenetic work of Bogler and Simpson, 1996. They note a close relationship and conclude that Dracaena might nest in Convallariaceae Horan., however, they do not re-draw the family boundaries from those of Dahlgren. Sansevieria they find to be close to Dracaena, Pleomele they do not find.
Bos, 1998 notes morphological similarities between Convallaria and Dracaena. This affinity pervades all later classifications.
Ruscus L.
We’re very familiar with Ruscus, particularly Butcher’s Broom, R. aculeatus, distinctive with its broad phylloclades fulfilling the photosynthetic role of leaves.
There is a consensus from accumulated phylogenetic evidence, presented and confirmed by Fay et al., 2000, Rudall et al., 2000, Chase et al., 2009, Kim et al., 2010 and others, that has Dracaenaceae sitting in a broadly defined Ruscaceae family. Kim et al., 2010 consolidates previous molecular analysis and finds very strong support for a monophyletic Ruscaceae. Fay et al., 2000 had referred to this group as the Convallariaceae Horan., however, Rudall. et al., 2000 quickly spotted that Ruscaceae Spreng. has priority.
All APG family arrangements are based upon a broadly drawn Ruscaceae which contains Dracaenaceae. Table 1 shows the morphological diagnosis which supports the molecular evidence for this monophyletic arrangement.
Table 1. Diagnostic morphology of Ruscaceae Spreng. nom. cons.
Morphology of the Ruscaceae
(Right click to open in a new tab)
Others
Several other genera have had big parts to play in the Dracaena story, there is not space to mention them all. Cordyline has often figured, vegetatively very similar, its multi-loculed ovules and phytomelan covered seeds distance it. Bos, 1998, on these and other characters placed it in the Lomandraceae, which has become Lomandroideae in the APG regime. Dasylyrion, Calibanus and Nolinia are also tree-like monocots, superficially similar, separated in earlier family systems, but now with Dracaena in the Asparagaceae / Nolinoideae, sensu APG III+. Liriope, Ophiopogon and Maianthemum all contribute and deserve greater recognition than has been given here.
Why Asparagaceae?

APG III Arrangement of the Asparagaceae, including Dracaenaceae.
This treatment is based upon Chase et al. 2009, savevfor the species numbers, WCSP.
Figure 3. shows the story line to the current APG III+ system. APG III circumscribes an expansive, undivided Asparagaceae, with the broadly circumscribed Ruscaceae, including Dracaenaceae, thereby becoming a subset of a very large, diverse and ill-defined assemblage of genera. APG III+ takes the framework of one consensus, and re-presents it in subfamily-of-Asparagaceae form, the Nolinoideae, as shown here. Were name conservation available at subfamilial level, the authors would have sought to call it Ruscoideae, Chase et al., 2009.
APG III+ “This is a highly unsatisfactory family. Nothing characterises it”. Some subfamilies are difficult to recognise, with Ophiopogon and Polygonatum juxtaposed to Dracaena, we could clearly include Nolinioideae in this candid self-analysis. Pégot-Ogier, E. (1871: 59), referring to the great and mythologically embued Dragon Tree of of Tenerife also had strong views on this arrangement, when it was proposed the first time:
“And now, ancient tree, first-born and most venerable of created beings, thou art dishonoured hopelessly, for it is in the name of science. O fabulous dragon, though who wert deified; thou art nothing more than an asparagus plant, an asparagus plant larger than any other, that is all! Thou art no longer a tree, the monarch of the forest; though art but mere vegetable, classed in the family asparageae, and this sentence is without appeal.”
It’s an indisputably morphologically indistinctive family assembled to minimise the number of smaller phylogenetically defined, morphologically indistinctive families that might monophyletically nest within it, APG III. In other words, having one confusing family is less confusing than several confusing families. A further reason is put forward to support inclusion in Asparagaceae, consistency with the “condensation of families that has already been made in Liliales”, (APG II, p. 404).
This arrangement was only “bracketed” in APG II, i.e. the authors recognised another reasonably held view that Ruscaceae and other smaller families could persist. And indeed they do, published, for example in Judd’s textbook (Judd et al., 2008), and in Seberg, in Heywood et al, .2007. “the expanded Ruscaceae preferred by APG II (2003) may be an ephemeral option”, wrote Seberg et al., 2012. Although their own molecular evidence does not contradict the monophyly of Aspapragaceae, including Ruscaceae, they are at odds with adopting it, rejecting the reasons APG III and APG III+ give for the changes as premature, and having neither morphological, phylogenetic nor philosophical imperatives.
The language of the birds … is the language of wisdom …
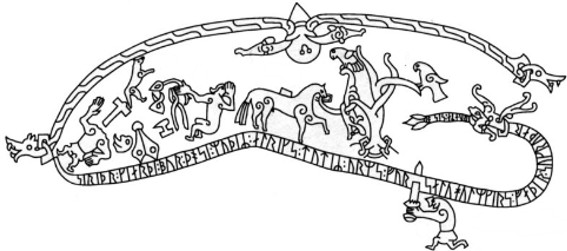
When Sigurd of medieval German myth, made famous as Siegfried in Wagner’s Ring Cycle, puts a Dragon’s blood-soaked finger to his mouth, the blood he tastes gives him understanding of the bird’s language; their warnings save his life. Caples, 1976.
A record of the power of a Dragon’s Blood. From Ramsund in Sweden, this early middle-age rock carving depicts Sigurd’s enlightenment bestowed by the taste of a Dragon’s Blood. (This image is in the public domain, source Wikipedia).
Much evidence and knowledge has accumulated and several chapters in the Dracaenaceae story already written, but readers should be forgiven for getting lost in the looping and swirling plot. Perhaps our Socotran Dragon’s Blood, that from Las Islas Canarias, or even the Dragon in the east, D cochinchinensis, may yet impart its precious virtue to a hero of the Dracaenaceae story. Whatever the source, we are greatly in need of such a rare gift of wisdom, so that it’s as yet hazy future can be revealed in a more measured manner.
Images
Images are copyright where indicated, and then used courtesy of Creative Commons licensing or permission of the owner.
References and further reading







































Hi,
Do you selling Getah Jernang ( Dragon`s Blood ) ? My factory can buy 500-2000kg every month with good price . If you are supplying it , please contact me my office in Singapore . Thank you !
Best Regards
Sean
Kakao Talk ID: HooplaHa
Skype ID : seanchapter
Email:seangao61@gmail.com
+65 81754357
Dear Sean,
No, we do not sell any Dragon Tree products. This post is for academic and educational interest only.
Kind regards,
Peter